Details of HEDOS CLINICAL TRIAL
DURATION
Study duration per patient: two weeks
Visit 1: Screening Visit (within 7-14 days before Visit 2)
Visit 2: Surgery and Randomization
Visit 3: Safety Observation (Day 1)
Visit 4: Safety Observation (Day 2)
Visit 5: Safety Observation in case of event (Day 1 after re-bleeding)
Visit 6: Safety follow-up in case of event/regular follow-up (Day 14)
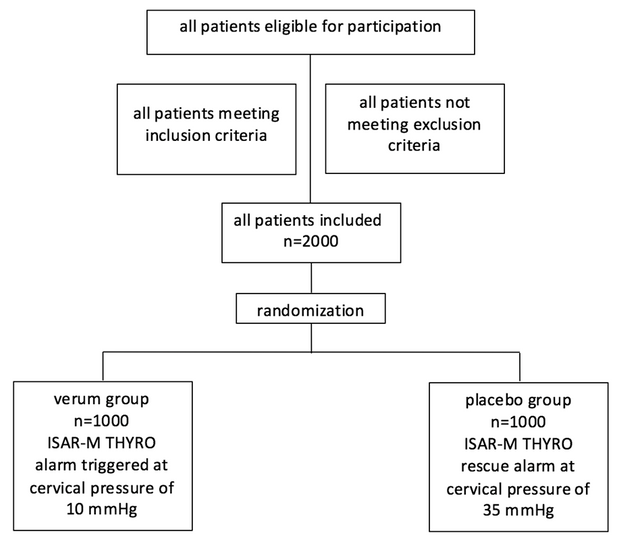
INCLUSION CRITERIA
To be eligible to participate in this clinical investigation, subjects must meet ALL of the following inclusion criteria:
Patients
- with indication for bilateral thyroid resection with suspected benign thyroid disease or -
- with indication for bilateral thyroidectomy with carcinoma and lymphadenectomy in central compartment 1 and / or 2 only or
- with indication for unilateral thyroidectomy but complete exploration of the contralateral thyroid lobe as complete mobilization in spatium chirurgicum contralaterally or
- with planned unilateral surgery (hemithyroidectomy, unilateral subtotal resection)
- Age of 18 years and above
- Signed written informed consent
EXCLUSION CRITERIA
Subjects shall not participate in this clinical investigation if they meet ANY of the following exclusion criteria:
- Children (< age of 18 years)
- Legally supervised people
- Endoscopic Surgery
- Lymphadenectomy of lateral compartments
- Contraindications for surgery
- Pregnancy
- Present participation in another study
STUDY PROTOCOL
The study protocol will be published as soon as it is approved by the local review board
Copyright 2017 @ HEDOS CLINICAL TRIAL STUDY GROUP All Rights Reserved